When the Covid-19 pandemic hit the UK in March 2020 the UK Government responded by launching its rapidly manufactured ventilator system initiative.
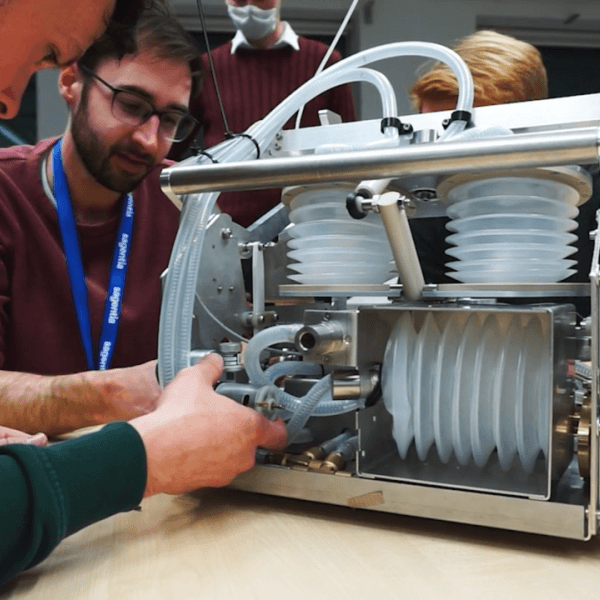
Cambridge based Sagentia worked to develop a new ventilator design suitable for rapid manufacture, in direct response to the UK Government’s “Ventilator Challenge”. The Sagentia team developed a largely mechanical ventilator, which is software-free, using a majority of custom-fabricated parts to avoid the potential for supply chain conflict around critical components. The design therefore lends itself readily to being manufactured and assembled globally.
Much to everyone’s relief, the Government announced that the NHS has not been overwhelmed in the manner that was first feared, therefore the manufacture of the Sagentia Ventilator, and the 4 other ventilator designs that also got through to the final stage, is not required in the UK at present.
Somersault partnered with Sagentia to capture the project story in this 3 minute film:
Achieved in the most spectacularly tight timescale. The Somersault team remotely interviewed over twenty key Sagentia stakeholders to piece together a film showcasing how in just 7 weeks they designed and fully documented a functional ICU ventilator, set up an ISO 13485-compliant assembly facility and established the necessary processes to support the assembly of >1,000 Sagentia Ventilator units per week. The Sagentia team drew on its experience of rapid medical product development and then sprinted faster than ever before to achieve what would have previously been thought impossible. Working 24/7 with a large multidisciplinary team, Sagentia completed a development that would normally take anywhere between 3- 5 years in a matter of weeks. A final design iteration underwent evaluation at an independent, MHRA-designated testing facility in early May and the Sagentia Ventilator was one of a number of designs described by expert clinicians as “clinically viable”.
Paul Wilkins, Managing Director of Science Group’s medical division said, “The commitment and ingenuity demonstrated by our team has been phenomenal. Huge sacrifices have been made by them and their families as they’ve worked tirelessly to bring the Sagentia Ventilator to clinical readiness. I am hugely proud of them and grateful for their efforts. I would also like to acknowledge the similar efforts made by many of our suppliers, working through the night and over weekends to fabricate the various parts we needed, as well as those of several other Cambridge-based consultancies who were working on their own designs in parallel. It has been a huge privilege to work on this project and we are grateful to the UK Government for the opportunity to play our part.”